The following information applies to the : CAL3K-AP , CAL3K-A , CAL3K-F.
The spiking method is called for when a sample has a very low CV or burns reluctantly. Then a better burning material is added to the sample to help the process. The better burning sample is the spike.
The ‘Spike’ must be of a known calorific value, usually benzoic acid of approx. 26.454MJ/Kg. On some occasions a Gelatine Capsule (CAL2K-4-GC) or Crucible Cover Disc (CAL2K-FLDS) needs to be as a Spike.
A Gelatine Capsule is used when burning:
- Fine powdery substances, which ‘scatter’ easily
- Benzoic acid powder
A crucible cover disk is used when burning:
- Volatile samples
Powdery substances can be "burned" without using the spiking method, but using the Gelatine Capsule option.
When using these items, you will first need to determine the energy value of the spike.
GELATINE CAPSULE FOR POWDERY SUBSTANCES: The gelatine capsule is the Spike.
CRUCIBLE COVER DISK FOR VOLATILE SUBSTANCES: The cover disk is the Spike.
BAD/RELUCTANT BURNING SUBSTANCES: The BA is the Spike.
BA POWDER IN A CAPSULE: The SUM OF BA and CAPSULE is the spike.
SPIKE VALUE
Depending on what the spike is you need to burn the spike material and determine its calorific value. If a Benzoic Acid tablet is the spike then the value is known. The CV of the gelatine capsule is also known, but the cover disc needs to be determined. This is best done by burning, let's say 10 cover discs and dividing the result by 10 to obtain the CV of a single disc. This obtained CV value must be entered as the SPIKE VALUE in the CAL3K.
SPIKE MASS
This is the mass of the substance to be used for spiking. The procedure to follow is to place the crucible on the balance and press TARE. Then add the substance to be used for spiking to the crucible. This Mass value is the “Spike Mass” and must be entered manually into the Calorimeter. Tare the balance again. Then add the sample. This mass is the sample mass and must be entered in as the Mass (Balance, F1, or manual).
STEP BY STEP ENTERING THE SPIKE MASS
- Place the crucible on the Balance.
- Press “Tare” to clear the weight of the crucible and get to 0.0000g
- Place one Benzoic Acid tablet (as a spike) and note the Mass = Spike Mass
- Enter the Spike mass by ‘Spike Mass’ enter, mass value
- Tare the balance
- Add the sample to the crucible and note the sample mass
- Enter the sample mass electronically (F5) or manual (F1)
- Place the prepared crucible (benzoic acid tablet with sample) in the lid assembly, inside the Vessel, close the Vessel and insert it in to the well.
- When using MANUAL FILLING, fill the vessel with oxygen BEFORE inserting it into the well.
- Enter the SID, e.g. 001
- Close the Lid of the Calorimeter
- The CAL3K Calorimeter will now perform some standard checks and if all is okay, it will begin with the “Initial Phase”, “Fire”, “Main” phase and then indicate that the determination has been completed.
Once the determination is complete, the result will display the calculated calorific value of the sample. The calorimeter will automatically take the end result, minus the spike energy and present the final result of your sample.
ENTER “SPIKE MASS”:
 |
Exit, Clear command entry |
 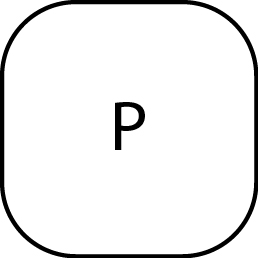 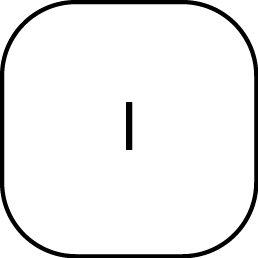     |
Start of command, spike mass |
 |
Accept Command |
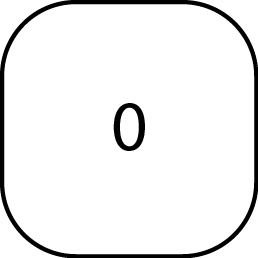   |
Enter spike mass value |
 |
Accept Value |
A ‘SPIKE MASS’ greater than zero indicates that the sample was spiked. The spike mass is set to zero after CV determination.
The ‘SPIKE VALUE’ is set to 26.454MJ as a default. It can be changed from the keyboard after the password is entered, by:
 |
Exit, Clear command entry |
       |
Start of command, spike value |
 |
Accept Command |
      |
Enter spike value |
 |
Accept Value |
Attaching the Firing Cotton to the Firing Wire
In this tutorial we will show you the correct way to attach the firing cotton to the firing wire when preparing a sample for firing in any of our CAL3K Oxygen Bomb Calorimeter Systems. The exercise is fairly simple and can be completed with or without the stainless steel tweezers.
Learn MoreThe Spiking Method in Bomb Calorimetry
What is spiking? If a sample does not ignite easily or not at all in a calorimeter, then the spiking method of ignition can be used to promote complete combustion of the sample. Only once the sample is completely burned can an accurate determination of the sample be ascertained.
Learn MoreThe use of Benzoic Acid in Bomb Calorimetry
Although benzoic acid has many uses outside of bomb calorimetry, we will touch on the subject of its use in calorimetry. Benzoic acid is relatively non-toxic and occurs naturally in plants. Appreciable amounts of it has been found in most berries (around 0.05%) and is formed in apples after infection with a specific fungus.
Learn More
