
How does a bomb calorimeter work?
In short, a small amount of the sample material is combusted in a closed container (The Bomb Vessel) which is filled with oxygen. The heat released by the sample is compared to a calibration (with a known calorific value) and the CV of the sample is calculated. The sample material must be combustible. It can be solid or liquid, but not a gas.
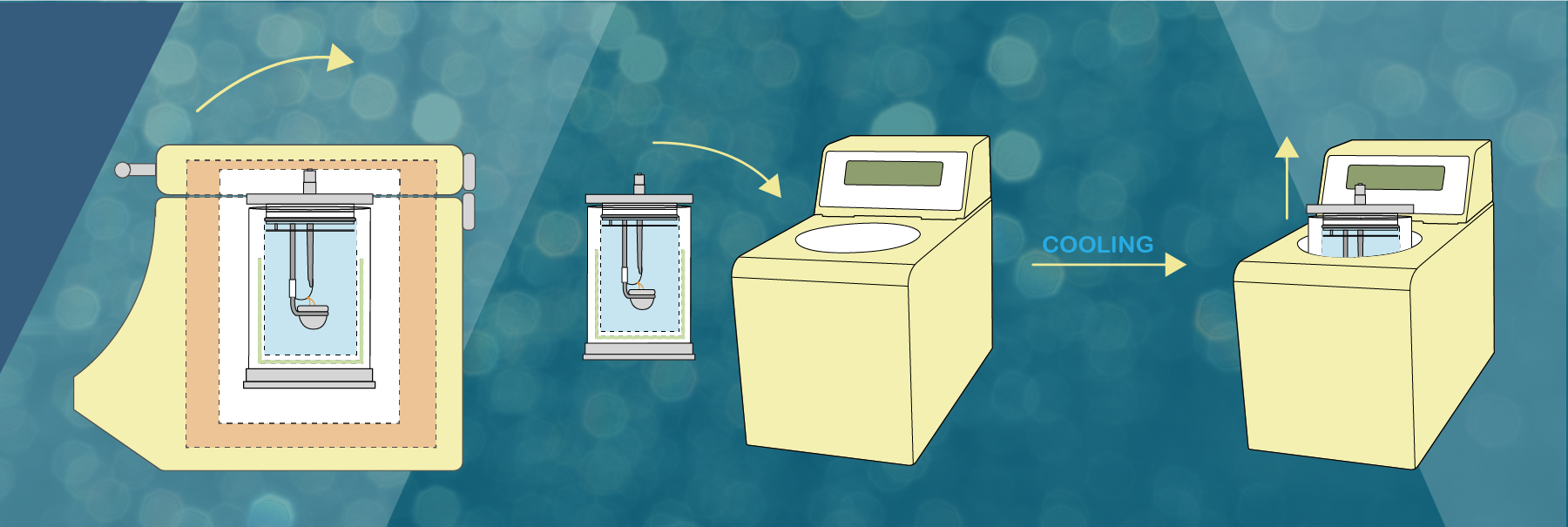
The DDS CAL3K range uses approx. 0.5g of sample mass for a determination. The exact amount (4 decimal places) is placed in a crucible, and the filled crucible is placed inside the bomb vessel.

The vessel is then filled with 30bar (435psi) oxygen and is placed in the calorimeter and the lid is closed.
The calorimeter takes over and after a while ignites the sample (inside the vessel) by sending a high current through the firing wire, which in turn ignites the sample.

Then the vessel temperature rises which is monitored by the unit every 6 seconds with a resolution of 0.000’001C. After 2-6 minutes a temperature equilibrium is obtained and the temperature rise is compared to a calibration temperature rise and the result is calculated.
Once the vessel is cooled it can be used again. Different CAL3K units have different timing procedures and different number of vessels, which influence the samples per hour determinations.
WATERLESS CALORIMETRY
Traditionally water is used in adiabatic and isothermal combustion calorimeters. It is used as a heat sink, as a transfer medium, or as a transport medium. In short : A calorimeter will combust a weighted sample in a steel cylinder and measures the resulting temperature increase. From the temperature increase it calculates the calorific value. Somewhere in the process...
Read MoreBOMB CALORIMETERS EXPLAINED
Combustion Calorimeters measure the heat released from a combustible solid-liquid substance. This is done by weighing a precise measure of the sample substance into a crucible, placing the crucible inside a "bomb" (a sealed metal cylinder called a vessel), filling the vessel with oxygen and igniting the substance.
Read More
