
Two of the most common types of calorimeters are the coffee cup calorimeter and the bomb calorimeter.
They might use the same calorimetry principles, but are very different in design and operation.

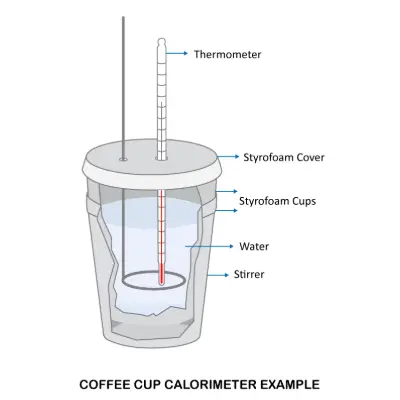
A coffee cup calorimeter is essentially a polystyrene (styrofoam) cup or two with a lid. The coffee cup calorimeter is operated at constant pressure (ambient pressure). The cup is partially filled with a known volume of water and a sensitive thermometer is inserted through the lid of the cup so that its bulb is below the water surface. This type of calorimeter is typically used for solution based chemistry with little to no volume change.
When a chemical reaction occurs in the coffee cup calorimeter, the water absorbs the heat of the reaction. Since the styrofoam cup insulates the reaction from the outside world (adiabatic), we can assume all the reaction's energy is absorbed into the water.
The change in the water temperature is used to calculate the amount of heat that has been absorbed (used to make products, so water temperature decreases) or evolved (lost to the water, so its temperature increases) in the reaction. The coffee cup calorimeter is often only used as the first calorimeter type experiment for a university student, due to its simplicity and low cost.
A coffee cup calorimeter is great for measuring heat flow in a chemical solution, but it can't be used for reactions, which involve gases since they would escape from the cup. The coffee cup calorimeter can't be used for high temperature reactions either, since these would melt the cup. A bomb calorimeter is used to measure heat flow for solids with low to high temperature reactions. Bomb calorimeters can not do a chemical solution reaction because the commencement of the reaction can not be controlled (triggered). Equally, a bomb calorimeter can not be used to fire gas samples.
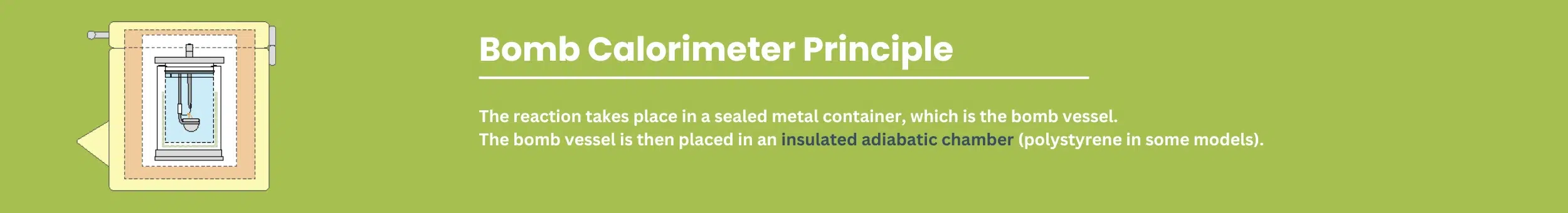
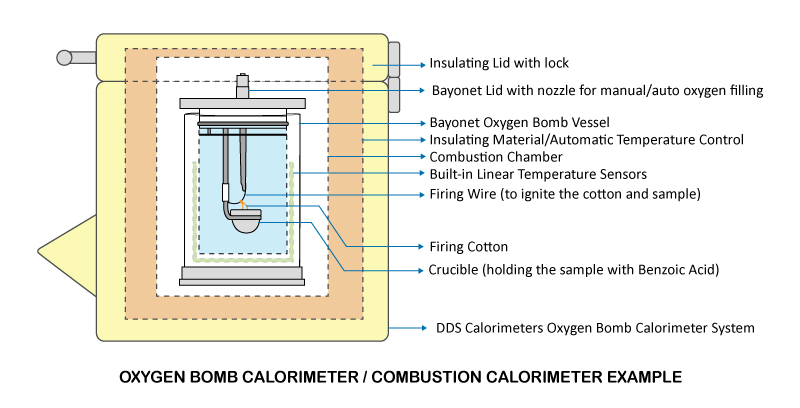
A bomb calorimeter works in the same manner as a coffee cup calorimeter, with one big difference. In a coffee cup calorimeter, the reaction takes place in the water. In a bomb calorimeter, the reaction takes place in a sealed metal container, which is the bomb vessel. The bomb vessel is then placed in an insulated adiabatic chamber (polystyrene insulation in some models).
The other major difference is that the bomb vessel is a sealed high-pressure vessel. The bomb vessel, with the sample inside, is inflated to 30 Bar with pure oxygen. The oxygen assists the burning process of the sample to guarantee a complete burn. This in turn provides for a consistent set of results.
When a sample is fired, heat of the reaction is totally absorbed into the bomb vessel walls. No energy is lost due to a sealed container and insulated by a polystyrene shell, just like the coffee cup calorimeter. The only mass (coffee cup had a known water mass) in the chamber is the constant mass of the bomb vessel, which we call the bomb factor.
The temperature difference of the bomb vessel is measured, just as it was for a coffee cup calorimeter. Due to the mass of the bomb vessel, time needs to be given for the reaction heat to be totally absorbed into the bomb, but still quicker than 80% of the calorimeters on the market. Our bomb vessel houses 8 high precision temperature sensors inside the bomb vessel walls, allowing for instantaneous quick and accurate temperature analysis.
The other difference of a bomb calorimeter is that the bomb vessel can be reused again and again, with a simple cooling cycle in between determinations. Typically our bomb calorimeter are able to run a sample every 6 minutes, so they are extremely fast and easy to set up.
IN CONCLUSION
In conclusion, the bomb calorimeter is more accurate and leaves less room for human error. Our bomb calorimeters use a digital temperature sensor with a 22 bit resolution versus an analogue glass tube thermometer used in a coffee cup calorimeter. It is faster and excludes environmental variables that may not have been considered. It also gives you the energy content of the substance being burned, which means that you do not have to calculate it.
If you plan to introduce the principles of calorimetry to students, then the coffee cup calorimeter is the correct option. It is cheap, simple to build and easy to explain. The bomb calorimeter is a serious instrument based on the same calorimetry principles as the coffee cup experiment. Comparatively, the bomb calorimeter calculates every aspect of the initialization, firing and final phase of the determination, continuously comparing the results against the calibration curve, providing a compensated result accurate to four decimal places.
Only a bomb calorimeter is able to provide Relative Standard Deviation (RSD), a measurement of determining the repeatable precision of a determination.



Can a bomb calorimeter calculate the enthalpy of fusion?